Research
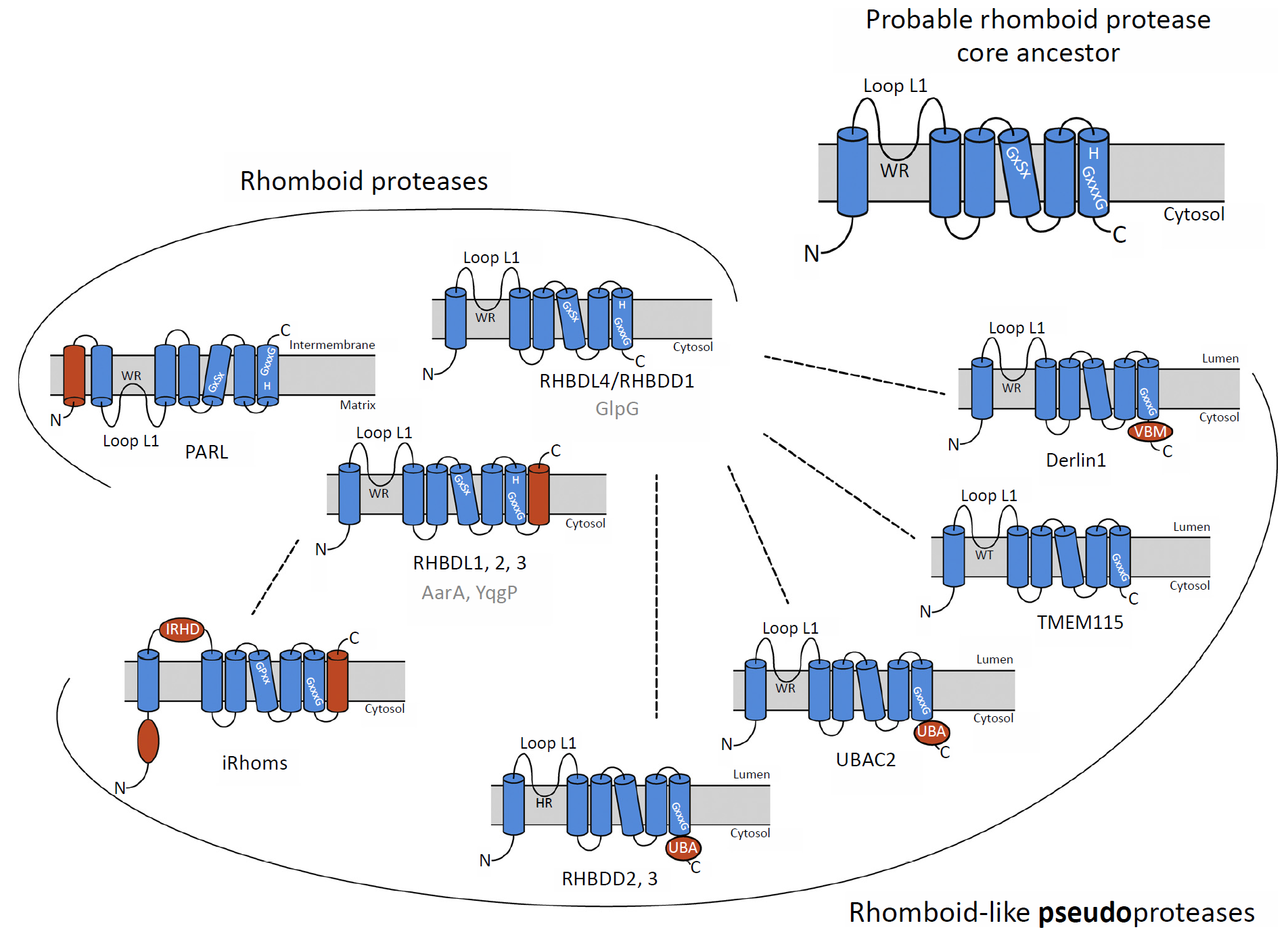
We are currently focusing on the nearly ubiquitous intramembrane proteases of the rhomboid family. Rhomboid proteases are known to regulate growth factor signaling in flies, mitochondrial dynamics in yeast or pathogenicity of the malaria parasite, and their proteolytically inactive cousins, rhomboid-like proteins, have been implicated in membrane protein trafficking and quality control. However, the biological functions of most rhomboid-family proteins and their molecular details remain poorly understood or unexplored. In our integrative approach we combine membrane biochemistry, enzymology and structural biology to understand how rhomboid proteases recognise and select substrates, and employ methods of quantitative proteomics, cell biology and genetics to uncover rhomboid functions in selected organisms. We are particularly interested in the basic biological aspects of intramembrane proteolysis relevant for biological signaling, membrane protein biogenesis and homeostasis, but we also exploit the acquired mechanistic insight in the development of rhomboid inhibitors.
The lab has been funded by the EMBO Young Investigator Programme, La Caixa Foundation, Marie Curie actions of the EU 7th Framework Programme, Czech Ministry of Education, Czech Science Foundation, Academy of Sciences of the Czech Republic, Charles University, and our home Institute.

